Cryoprotents
are used in the solution that the embryos are frozen in. There
are 2
basic types - permeating (e.g. propanediol) and extracellular, such
as sucrose and lipoprotein (egg yolk). Cryoprotents are useful
because they:
 |
Lower freezing point and may prevent
intracellular ice formation
until temp very low.
|
 |
May protect
cells by interacting with membranes as they change
from
a pliable to a rigid state.
|
Embryos can be frozen at the pronuclear stage (one cell), or
at any stage after that up to and including the blastocyst
stage (5-7
days after fertilization).
Different cryoprotents are used for different stages of
embryo development.
Embryo survival rates after thawing and pregnancy rates in most
IVF programs are highest for embryos that were frozen at the pronuclear
stage, or at the 2-cell to 4-cell stage.
How are frozen-thawed embryo transfer
cycles managed?
There are many different protocols for both "natural cycle" and for "hormone
replacement cycle" thawed embryo transfers. Following are examples for each.
There is nothing magic about these protocols - these are just examples for educational
purposes. At the Advanced Fertility Center of Chicago we usually use hormonally
replaced cycles.
Natural cycle
 |
Ultrasound until the dominant follicle is
greater than 14 mm in mean diameter. |
 |
Then, daily urine LH testing. |
 |
Once ovulation confirmed by LH surge, thawed embryo transfer
is planned for
3 days after LH surge. |
 |
Pronuclear embryos are thawed the day before transfer,
cleaved embryos are thawed on the day of transfer or the
day before. |
 |
Luteal support: 200 mg vaginal suppositories can be used
twice daily. |
|
Pregnancy test 12-14 days after transfer. |
Hormone replacement cycle
 |
GnRH agonist (e.g. Lupron or Synarel)
is given, either midluteal (such as day 21) or very early
follicular phase, such as day 2.
|
 |
Down-regulation
is confirmed by ultrasound and blood tests about 10 days
later
|
 |
Estradiol valerate
2 mg twice daily is started once endometrium <4 mm
and no follicular activity seen on ultrasound. This dose
may
need to be increased. |
 |
When the endometrium
is satisfactory in thickness and reflectivity, progesterone
is started.
|
 |
Embryo transfer is planned for 3-6 days
later - depending on the stage of development of the embryos
to be replaced. |
|
|
The same estrogen and progesterone doses
are continued in the luteal phase.
|
 |
Pregnancy testing is done 8-14 days
after transfer - depending on the stage of development of
the embryos replaced. |
 |
If pregnant, estrogen and progesterone
are continued until at least 12 weeks and then weaned off
gradually. |
There are other protocols that use transdermal estrogen patches
or various other methods of progesterone support, etc.
"Window of implantation"
Implantation in some other mammals
There are some very interesting variations among different mammalian
species.
" Delayed implantation", also called embryonic diapause
has been described in about 100 species of mammals.
Ovulation - mating - fertilization - and subsequent development
to the blastocyst stage occurs. The blastocyst then remain in
uterus without implanting or developing further. In some species,
the corpus luteum in the ovary is later reactivated at which
time the embryo implants and continues development.
The swamp wallaby, a marsupial, is a great example:
This animal mates during pregnancy: About 4-6 days before giving
birth.
The sperm enter the non-pregnant uterus (this animal has a double
uterus), and the egg is fertilized.
The resulting embryo develops to the blastocyst stage
and then goes into "diapause" (like hibernation).
The mother gives birth to the pregnancy that was near
the end, and, after the young are finished suckling,
the blastocyst
that
is in diapause in the other uterus "wakes-up" and
implants, develops, etc.
Implantation in humans
The
concept of a "window" of implantation:
After sufficient estrogenic exposure, initiation
of progesterone initiates a "clock" that results in the uterine lining
passing through a receptive "window" of time when implantation
can occur. Before, or after the window - implantation will not
occur.
Rosenwaks et al, in 1987 published an excellent article that
looked at donor embryo transfers done in natural cycles. They
got good results when transferring 4-6 cell embryos on day 17-19
endometrium (day of LH surge was called day14).
Formigli et al, in 1987 reported uterine lavage of embryos from
uteri of donors at 5 days post-ovulation. The embryos were then
transferred to recipient women. They had pregnancies when the
recipient's cycle was from 4 days in front of to 3 days behind
the donor's at ovulation. This suggests a window of implantation
of up to 7 days.
Navot et al, in 1991 reported on donor embryo transfers done
with 2-3 day old embryos on recipient "cycle days" 15-20
(artificial cycles). Pregnancies resulted from transfers on all
days. This suggests (at least) a 6 day transfer window.
The "window of transfer" is a little different from
the window of implantation. A 2-3 day old embryo takes 3-4 days
to become a blastocyst. Blastocysts can hatch and implant. Therefore,
from all of the above information, the inferred window of implantation
may extend from days 18-19 to 23-24 of the "idealized cycle".
Embryo Cryopreservation
In vitro
fertilization (IVF) is a remarkable diagnostic and treatment
tool for infertile couples. Since the birth of the
first IVF baby in July 1978, IVF has become the ultimate treatment
for almost every cause of infertility. Nevertheless, any single
embryo transfer has a finite probability for success. Consequently,
one of the important strategies of IVF treatment, similar to
natural attempts at conception, is to keep trying. “Most
of the important things in the world have been accomplished
by people who have kept on trying when there seemed to be no
help
at all.” - Dale Carnegie (1888–1955), US author
of How to Make Friends & Influence People.
With many IVF cycles there is help to keep trying and it comes
in the form of frozen excess embryos. Embryo cryopreservation
has its roots in the accidental successful cryopreservation of
fowl sperm in 1948. Scientists apparently mislabeled the experimental
freezing solutions and used glycerol instead of another compound.
The glycerol solution was highly effective and its use led to
a new branch of science. Subsequently, several solutions have
been identified that protect the cells or tissues during the
freezing process. Interestingly, cryopreservation of biological
material has had its greatest practical impact in the field of
reproduction. Cells and tissues freeze successfully and for very
long periods of time. The process of freezing cells and tissues
involves cryoprotents that prevent the build up of salts as
water crystallizes during freezing. High concentrations of salts
and perhaps the ice crystals themselves can mortally wound cells
either during freezing or thawing. The cells (embryos in this
case) are stored in liquid nitrogen after a controlled freeze
by special machinery. Embryo freezing takes several hours while
the thaw process takes about 30-45 minutes. Embryos have been
successfully thawed after cryopreservation for as many as 13
years. Clinical pregnancies have been reported from embryos stored
for 9 years.
Meticulous attention to detail and careful adherence to guidelines
are required to achieve embryo survival and viability that
results in live born babies. “It has long been an axiom of mine
that the little things are infinitely the most important.” -
Sir Arthur Conan Doyle, writer of Sherlock Holmes mysteries.
At Genetics & IVF Institute, our policy has been to freeze
each embryo in its own straw. Many clinics put 3-4 embryos
in the same cryopreservation vial. While our strategy is more
labor
intensive, it has important advantages for patients. First,
we know the quality of the embryo at the time it was frozen.
We
can use that information to decide the order to thaw the embryos.
Secondly, we can immediately judge the viability of each embryo
at the time of thawing so that we thaw only the number of embryos
that we want to transfer, avoiding embryo wastage.
Our strategy of individual freezing and thawing is critical
to achieve the exact number of embryos desired for transfer.
The alternative strategy often results in too many embryos
transferred with increased multiple gestations or too few embryos
transferred
and lower pregnancy rates. Since we know the pre-freeze characteristics
of each embryo frozen, and the embryo score is related to the
probability of implantation, we can purposely thaw and transfer
more embryos when the embryo quality is lower without concern
for high multiple gestation rates while maximizing each embryo’s
potential for implantation. We believe this strategy provides
the greatest efficiency and use of embryos in a frozen transfer
cycle.
Embryo cryopreservation also has enormous potential to avoid
ethical dilemmas for many couples. We have three basic options
for handling excess embryos. The embryos may be discarded, donated
anonymously to other infertile couples or donated to scientific
research. IVF couples choose one of these three options at the
time of egg retrieval but their choice may be changed any time
in the future. Embryo cryopreservation provides an opportunity
to use every embryo produced without discarding them. Some infertile
couples are uncomfortable with any of these choices and they
choose to inseminate only a few eggs and then transfer all resulting
embryos. Other couples continue to transfer their cryopreserved
embryos in subsequent frozen embryo transfer cycles with the
possibility of producing additional children. Overall, embryo
cryopreservation avoids many of the ethical dilemmas inherent
in producing a large number of embryos.
An uncommon but serious complication of IVF is ovarian hyperstimulation
syndrome (OHSS). The high hormone levels cause water to leak
from the ovarian blood vessels into the abdomen. If the woman
becomes pregnant, the syndrome often is much more severe and
lasts longer. Cryopreservation of all embryos in an IVF cycle
to avoid conception in a woman at risk for OHSS also can help
avoid a very serious medical illness.
Successful cryopreservation of excess embryos is an increasingly
important tool to reduce the number of multiple gestations resulting
from infertility treatments. Over time, infertility treatments
have become more successful and efficient in producing pregnancies.
As such, the potential for individual embryos to become babies
(implantation rate) has increased. The worldwide trend is to
transfer fewer embryos, which results in more embryos for freezing.
The question on most couples minds when they contemplate a frozen
embryo transfer (FET) cycle is how many embryos will survive
the thaw process and what are my chances for pregnancy?
The factors that predict pregnancy are complex and interrelated.
We recently evaluated our data and found that, when about 20
factors were introduced into a mathematical equation, the predictive
value was approximately 10%. Thus 90% of the factors predictive
of pregnancy are not observable by the medical staff. Nevertheless,
we rely on some clinical factors to help us decide which embryos
to thaw and to estimate the likelihood of embryo survival.
We define embryo survival based on the number of viable cells
in
an embryo after thawing. An embryo has “survived” if >50%
of the cells are viable. We consider an embryo to “partially
survive” if <50% of its cells are viable and to be “atretic” if
all the cells are dead at thaw. Approximately, 65-70% of embryos
survive thaw, 10% partially survive and 20-25% are atretic.
Our data suggests that embryos with 100% cell survival are
almost
as good as embryos never frozen but only about 30-35% survive
in this fashion.
Embryo morphology (appearance of the cells / percentage of fragmentation)
is one of the most influential factors for embryo survival. Interestingly,
embryos produced from intracytoplasmic sperm injection (ICSI)
also seem to survive somewhat better than embryos produced from
conventional insemination. The following graph illustrates these
points. The embryo grade in the graph goes from worst (3.2) to
best (1.0).
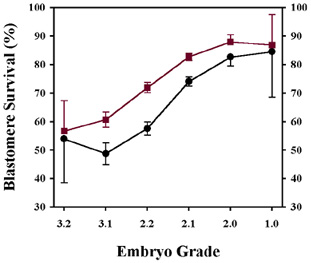
Embryos that are 2, 4 or 8 cells when frozen have about 5-10%
greater survival than embryos with an odd number of cells.
Donor egg embryos have a 2-5% greater survival rate than embryos
from infertile women when compared by morphology score.
Pregnancy rates are similarly affected by complex relationships
and like embryo survival only 7-10% of the predictive value
can be observed and measured. Age is not a significant factor
with frozen embryos but many fewer older women have frozen
embryos. From the approximately 20 factors reviewed, the
most important factors predicting pregnancy rates are the
number of surviving embryos transferred, the number of 100%
surviving embryos transferred and the morphology scores of
the transferred embryos. The delivered pregnancy rates ranged
from 5% (a single poor quality embryo) to 36% (4 high quality
embryos) when the cycles from 1987 to 2001 were combined.
Blastocysts (embryos cultured for 5 days rather than 2-3)
are a special case. The embryos are much larger and have
special needs with regard to freezing without damage. Many
centers have had trouble with blastocyst cryo-survival and
pregnancy rates. A new protocol developed in our laboratory
and implemented in December 2000 led to a transfer rate of
62% and a 35% pregnancy rate per transfer. This important
change now makes blastocyst transfer more appealing since
excess blastocysts can be expected to yield pregnancy rates
comparable to embryos frozen two to three days after retrieval.
A happy note to couples that have the opportunity to use
frozen embryos is the children are healthy and normal. Many
studies have evaluated the children born from frozen embryos
(“frosties”). The result has uniformly been positive
with no increase in birth defects or development abnormalities.
In summary, embryo cryopreservation adds an important dimension
to assisted reproduction. It extends the possibility for
pregnancy when fresh cycles fail or when couples want additional
children after a successful embryo transfer. Cryopreservation
helps avoid many ethical dilemmas by eliminating the need
to dispose of embryos for couples unwilling to donate them
to other couples or to scientific investigation. It also
offers an alternative to couples that might transfer too
many embryos and risk a multiple gestation pregnancy. Our
policy to freeze embryos individually provides a critical
feature for efficient use of the embryos. Embryo cryopreservation
adds about 10-30% more pregnancies per retrieval cycle and
the outcomes of the children are normal.
Excerpt from Genetics & IVF Institute
Embryo's Alive
(see paperwork)
Cincinnati, Ohio 45242-5020
E-mail: EmbryosAlive@Gmail.com
Hours 9:30 to 4:30 Monday–Friday Eastern Standard Time
Phone: 513-518-7006 Fax: 727-489-2427
|

